The Chemistry of Silly String
Whether you're spraying Silly String in a hunt for tripwires, at a celebration or just for fun, you're probably not thinking too much about its physical properties. But those properties are responsible for everything it can do, and taking a moment to define them can help you understand how the aerosol string works.
Silly String starts as a liquid inside the can and becomes continuous string outside of the can. The string is lightweight and slightly adhesive; it can stick to walls, clothing and other surfaces. It's also cohesive; unless you physically pull it apart, it holds itself together in one long piece. Most importantly, its adhesive strength is less than its cohesive strength. In other words, it takes less force to pull a strand off the wall than it takes to pull it apart. That's why you can usually grab Silly String after it dries, and the bulk of it will come off a surface in one big tangle. Exceptions are if you spray it at very close range, onto highly textured surfaces or onto surfaces that interact with its ingredients, like vinyl. In those cases, cleanup can require substantially more effort.
Advertisement
All of these properties come from Silly String's ingredients and the way they interact when extruded through the nozzle of a normal aerosol can. They create foam surrounded by a sturdy skin. The foam is light, and the skin is flexible and tacky. The foam and its skin come from three primary ingredients:
- A resin forms the plastic structure of the strand. The original formula used polyisobutyl methacrylate, an acrylic resin. Acrylic resins are also components in materials like Lucite and Plexiglas.
- A surfactant, or surface-active agent, helps the resin foam and modifies the stickiness of the strands. Wham-O's patent lists sorbitan trioleate as its surfactant. You can see the foaming action of surfactants in ordinary detergents around your home, and you can learn more about them in How Play-Doh Works.
- A propellant forces the other ingredients out of the aerosol can, causes them to foam and helps form the skin. The propellant described in the patent is dichlorodifluoromethane, also known as Freon-12. The Silly String manufactured today uses other propellants -- Freon-12 is an ozone depleting substance that is no longer manufactured.
Silly String's characteristic texture and shape come from the proportions of these ingredients. Resin is the string's plastic component -- it creates the physical framework for the strands. With too much of it, the liquid couldn't foam very much and the final product would have a texture more like caulk. With too little resin, the strands would be too fragile to hold themselves together.
Surfactant helps the resin foam and expand. Too much surfactant would make too much foam, and you'd end up with a texture more like shaving foam or mousse. But too little surfactant would make the strands too sticky, and you wouldn't be able to remove them from surfaces.
Neither resin nor surfactant is Silly String's primary ingredient, though. The plastic resin is only 10 or 15 percent of the mix, and the surfactant is less than five percent. By a substantial margin, most of what's in the can is propellant. The propellant causes the reaction between the resin and the surfactant. It's a lot like running a jet of hot water into a sink with dishwashing liquid -- the agitation from the jet of water and the presence of the surfactant combine to make foam.
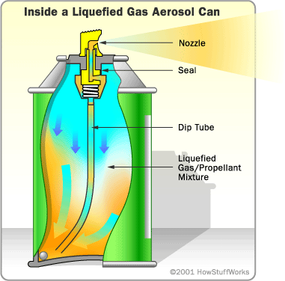
Without the propellant and the aerosol can, Silly String would have no foam. At normal air pressure, the propellant in Silly String would be a gas. The high pressure inside the can, however, keeps it in its liquid state, and all of the other ingredients are dissolved in it. When you press the nozzle, a valve opens inside the can, giving the highly compressed liquid a chance to escape. Liquid rapidly moves up the tube inside the can, traveling from the high-pressure environment inside to the lower-pressure environment outside. Outside of the confines of the can, the liquid propellant quickly becomes a gas, causing the remaining ingredients to foam. You can read more about aerosol cans in How Aerosol Cans Work.
In addition to making the foam itself, the propellant helps make the skin on the foam. As the propellant evaporates from the foam, the outer edge dries out, leaving a thin, flexible skin. This skin holds the Silly String together and allows it to stick to surfaces. If you spray Silly String onto a surface at very close range, the propellant does not have time to evaporate -- the resulting strand is wet and brittle. This property also explains why Silly String is a little puffier just after you spray it than after it dries. As the propellant evaporates from the spaces in the foam, the string collapses a little and becomes more compact.
The movement of propellant is central to the physical qualities of Silly String. Unfortunately, it has also lead to safety and environmental concerns. We'll look at those in the next section.
