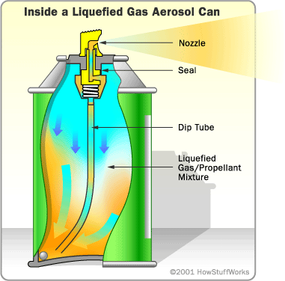
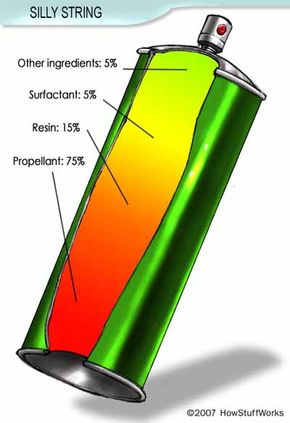
In December 2006, news agencies reported that American and British troops in Iraq had developed an inventive method for finding traps. By spraying Silly String down halls or through doors, people could see otherwise invisible tripwires. Since these wires could trigger explosive devices, Silly String quickly gained a reputation for being a lifesaver.
The trap-detection story piqued the HowStuffWorks staff's curiosity about Silly String itself. What does it take to make strands of material that are cohesive enough to loop over thin wires without breaking, but light enough not to spring the traps? How can a relatively small aerosol can filled with liquid produce hundreds of feet of a stringy solid? Why can the strands fly as far as they do? And who invented it in the first place?
Advertisement
A little research revealed virtually identical answers all over the Web. Sites asserted that Silly String was a complete enigma. Other than its manufacturer, no one knew what it was made of or how it worked. Some even claimed that the Silly String mystery was too tough for scientists to crack and that chemists could offer only their best guesses. But one fact remained fairly consistent -- according to news articles and Web sites, Julius Samann introduced the product in 1969.

A 1972 United States Patent for a "foamable resinous composition" disagrees with all of that. It describes an aerosol liquid that becomes a slightly tacky string when sprayed -- Silly String. According to the patent, the product's inventors are Robert P. Cox and Leonard A. Fish. The United States Patent and Trademark Office (USPTO) assigned the patent not to Julius Samann, but to Wham-O Manufacturing Company, maker of the Hula Hoop and the Frisbee. The Wham-O Web site agrees with the patent; according to its FAQ, it introduced Silly String in 1972.
Samann, on the other hand, appears nowhere on the patent. In fact, a search of all U.S. patents issued since 1790 reveals that Samann's name appears on exactly two patents. Both describe a "container for volatile substances." The volatile substances are perfumes; the containers are cardboard cut-outs. In one patent, the cut-out is shaped like a fir tree. In the other, it's the silhouette of a woman who appears to be in a state of undress.
So why do so many Web sites claim that Julius Samann, inventor of the tree-shaped car air freshener, introduced Silly String? The answer appears to lie in Silly String's more recent history. According to the USPTO's assignment information, Wham-O transferred the rights to the Silly String trademark to Julius Samann, Ltd. in 1999. Julius Samann, Ltd. owns Car-freshner Corporation, maker of the fragrant, dangling trees. Car-freshner's toy division, Just for Kicks, now makes Silly String. Other companies also make similar products with names like Fun Streamer and Crazy String.
In spite of the Internet lore, Silly String is not an impenetrable mystery. Everything about it, from the way it flies across the room to its knack for clinging to surfaces, comes from the interactions between its ingredients. We'll look at how ordinary chemicals become extraordinary liquid string in the next section.